summary: a new stem cellphone remedy approach eliminates centered brain tumors and offers long-term immunity, practising the immune equipment to evade cancer from returning.
source: Brigham and ladies's clinic
Scientists are harnessing a new manner to show cancer cells into powerful, anti-melanoma agents.
within the latest work from the lab of Khalid Shah, MS, Ph.D., at Brigham and ladies's health center, a founding member of the Mass ordinary Brigham healthcare device, investigators have developed a new phone therapy method to eliminate based tumors and result in lengthy-term immunity, working towards the immune equipment so that it may possibly steer clear of cancer from routine.
The crew confirmed their twin-motion, cancer-killing vaccine in an advanced mouse model of the lethal mind melanoma glioblastoma, with promising outcomes.
Findings are published in Science Translational medicine.
"Our crew has pursued an easy theory: to take cancer cells and seriously change them into cancer killers and vaccines," observed corresponding creator Khalid Shah, MS, Ph.D., director of the center for Stem telephone and Translational Immunotherapy (CSTI) and the vice chair of analysis in the branch of Neurosurgery on the Brigham and school at Harvard clinical faculty and Harvard Stem mobile Institute (HSCI).
"the usage of gene engineering, we're repurposing melanoma cells to enhance a therapeutic that kills tumor cells and stimulates the immune system to both spoil basic tumors and forestall melanoma."
melanoma vaccines are an active area of research for many labs, but the method that Shah and his colleagues have taken is distinctive. in its place of the usage of inactivated tumor cells, the crew repurposes residing tumor cells, which possess an unusual characteristic. Like homing pigeons returning to roost, residing tumor cells will travel long distances across the brain to come to the web site of their fellow tumor cells.
Taking competencies of this unique property, Shah's team engineered residing tumor cells using the gene editing device CRISPR-Cas9 and repurposed them to release tumor telephone killing agent.
moreover, the engineered tumor cells have been designed to specific factors that might make them easy for the immune device to spot, tag and be aware, priming the immune gadget for a protracted-time period anti-tumor response.
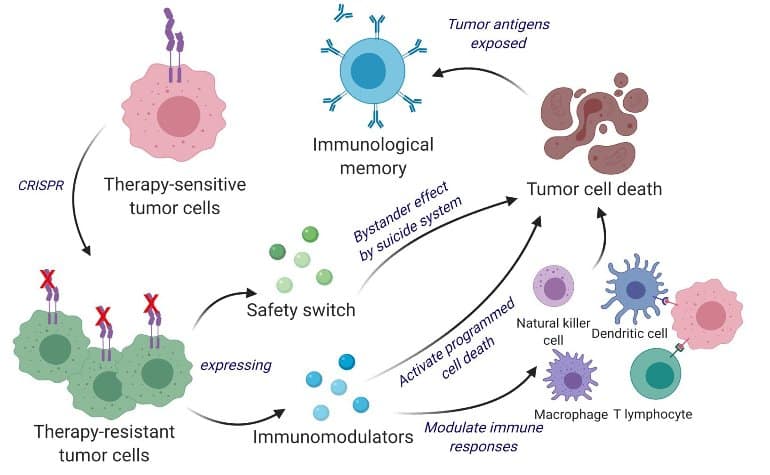 Scientists developed a bifunctional therapeutic strategy with the aid of remodeling residing tumor cells right into a therapeutic. Shah's team engineered residing tumor cells using the gene modifying tool CRISPR-Cas9 and repurposed them to unencumber tumor phone killing agent. furthermore, the engineered tumor cells were designed to specific components that would make them effortless for the immune device to identify, tag and remember, priming the immune system for a long-time period anti-tumor response. The crew tested their repurposed CRISPR-improved and reverse-engineered therapeutic tumor cells (ThTC) in distinctive mice strains including the one that bore bone marrow, liver and thymus cells derived from humans, mimicking the human immune microenvironment. Shah's crew additionally built a two-layered defense switch into the melanoma cell, which, when activated, eradicates ThTCs if necessary. credit score: Kok Siong Chen and Khalid Shah.
Scientists developed a bifunctional therapeutic strategy with the aid of remodeling residing tumor cells right into a therapeutic. Shah's team engineered residing tumor cells using the gene modifying tool CRISPR-Cas9 and repurposed them to unencumber tumor phone killing agent. furthermore, the engineered tumor cells were designed to specific components that would make them effortless for the immune device to identify, tag and remember, priming the immune system for a long-time period anti-tumor response. The crew tested their repurposed CRISPR-improved and reverse-engineered therapeutic tumor cells (ThTC) in distinctive mice strains including the one that bore bone marrow, liver and thymus cells derived from humans, mimicking the human immune microenvironment. Shah's crew additionally built a two-layered defense switch into the melanoma cell, which, when activated, eradicates ThTCs if necessary. credit score: Kok Siong Chen and Khalid Shah. The crew proven their repurposed CRISPR-better and reverse-engineered therapeutic tumor cells (ThTC) in distinct mice traces together with the one which bore bone marrow, liver and thymus cells derived from people, mimicking the human immune microenvironment. Shah's crew also constructed a two-layered defense swap into the cancer telephone, which, when activated, eradicates ThTCs if crucial.
This dual-motion mobilephone remedy become secure, applicable, and efficacious in these fashions, suggesting a roadmap towards remedy. whereas further trying out and development is required, Shah's crew in particular selected this mannequin and used human cells to easy the course of translating their findings for patient settings.
"all over all of the work that we do within the core, even when it's incredibly technical, we on no account lose sight of the affected person," talked about Shah.
"Our goal is to take an innovative but translatable method so that we can boost a therapeutic, cancer-killing vaccine that sooner or later can have a long-lasting impact in drugs."
Shah and colleagues notice that this therapeutic approach is relevant to a wider latitude of solid tumors and that extra investigations of its functions are warranted.
writer: Press OfficeSource: Brigham and ladies's HospitalContact: Press office – Brigham and ladies's HospitalImage: The photo is credited to Kok Siong Chen and Khalid Shah
common research: Open entry."Bifunctional melanoma telephone-based vaccine concomitantly drives direct tumor killing and antitumor immunity" by using Kok-Siong Chen et al. Science Translational drugs
abstract
Bifunctional cancer phone-based vaccine concomitantly drives direct tumor killing and antitumor immunity
The administration of inactivated tumor cells is established to set off a powerful antitumor immune response; despite the fact, the efficacy of such an approach is restricted by way of its lack of ability to kill tumor cells earlier than inducing the immune responses. unlike inactivated tumor cells, dwelling tumor cells have the ability to track and target tumors.
right here, we developed a bifunctional entire cancer mobile–primarily based therapeutic with direct tumor killing and immunostimulatory roles. We repurposed the tumor cells from interferon-β (IFN-β) sensitive to resistant using CRISPR-Cas9 through knocking out the IFN-β–selected receptor and consequently engineered them to release immunomodulatory brokers IFN-β and granulocyte-macrophage colony-stimulating factor.
These engineered therapeutic tumor cells (ThTCs) eliminated based glioblastoma tumors in mice through inducing caspase-mediated melanoma cellphone apoptosis, down-regulating melanoma-linked fibroblast-expressed platelet-derived increase element receptor β, and activating antitumor immune mobile trafficking and antigen-specific T cellphone activation signaling.
This mechanism-primarily based efficacy of ThTCs translated into a survival advantage and lengthy-time period immunity in basic, recurrent, and metastatic melanoma fashions in immunocompetent and humanized mice. The incorporation of a double kill-swap comprising herpes simplex virus–1 thymidine kinase and rapamycin-activated caspase 9 in ThTCs ensured the security of our strategy.
Arming naturally neoantigen-rich tumor cells with bifunctional therapeutics represents a promising telephone-based mostly immunotherapy for solid tumors and establishes a road map toward scientific translation.

Post a Comment